Zanello G, Goethel A, Rouquier S, Prescott D, Robertson SJ, Maisonneuve C, Streutker C, Philpott DJ, Croitoru K.
J Immunol. 2016 Jul 1;197(1):345-55. doi: 10.4049/jimmunol.1600185. Epub 2016 May 20.
http://www.ncbi.nlm.nih.gov/pubmed/27206769
Abstract
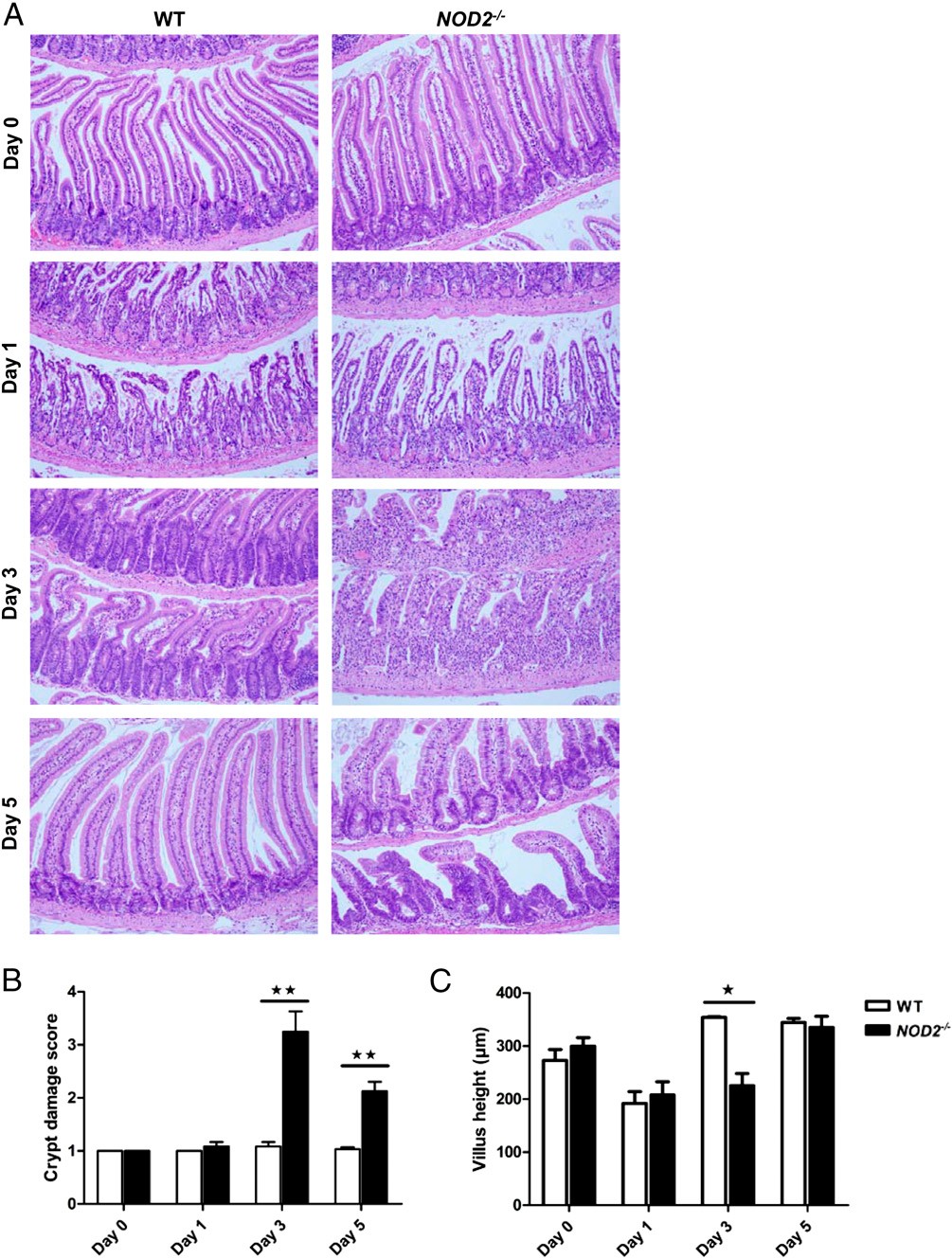
Loss of function in the NOD2 gene is associated with a higher risk of developing Crohn's disease (CD). CD is characterized by activation of T cells and activated T cells are involved in mucosal inflammation and mucosal damage. We found that acute T cell activation with anti-CD3 mAb induced stronger small intestinal mucosal damage in NOD2(-/-) mice compared with wild-type mice. This enhanced mucosal damage was characterized by loss of crypt architecture, increased epithelial cell apoptosis, delayed epithelial regeneration and an accumulation of inflammatory cytokines and Th17 cells in the small intestine.
Partial microbiota depletion with antibiotics did not decrease mucosal damage 1 d after anti-CD3 mAb injection, but it significantly reduced crypt damage and inflammatory cytokine secretion in NOD2(-/-) mice 3 d after anti-CD3 mAb injection, indicating that microbial sensing by Nod2 was important to control mucosal damage and epithelial regeneration after anti-CD3 mAb injection. To determine which cells play a key role in microbial sensing and regulation of mucosal damage, we engineered mice carrying a cell-specific deletion of Nod2 in villin and Lyz2-expressing cells. T cell activation did not worsen crypt damage in mice carrying either cell-specific deletion of Nod2 compared with wild-type mice.
However, increased numbers of apoptotic epithelial cells and higher expression of TNF-α and IL-22 were observed in mice carrying a deletion of Nod2 in Lyz2-expressing cells. Taken together, our results demonstrate that microbial sensing by Nod2 is an important mechanism to regulate small intestinal mucosal damage following acute T cell activation.
Copyright © 2016 by The American Association of Immunologists, Inc.